Published EMA Slideshow presentation containing 28 slides about general principals, advice and examples for presenting information in the SmPC of fixed combination medicinal products



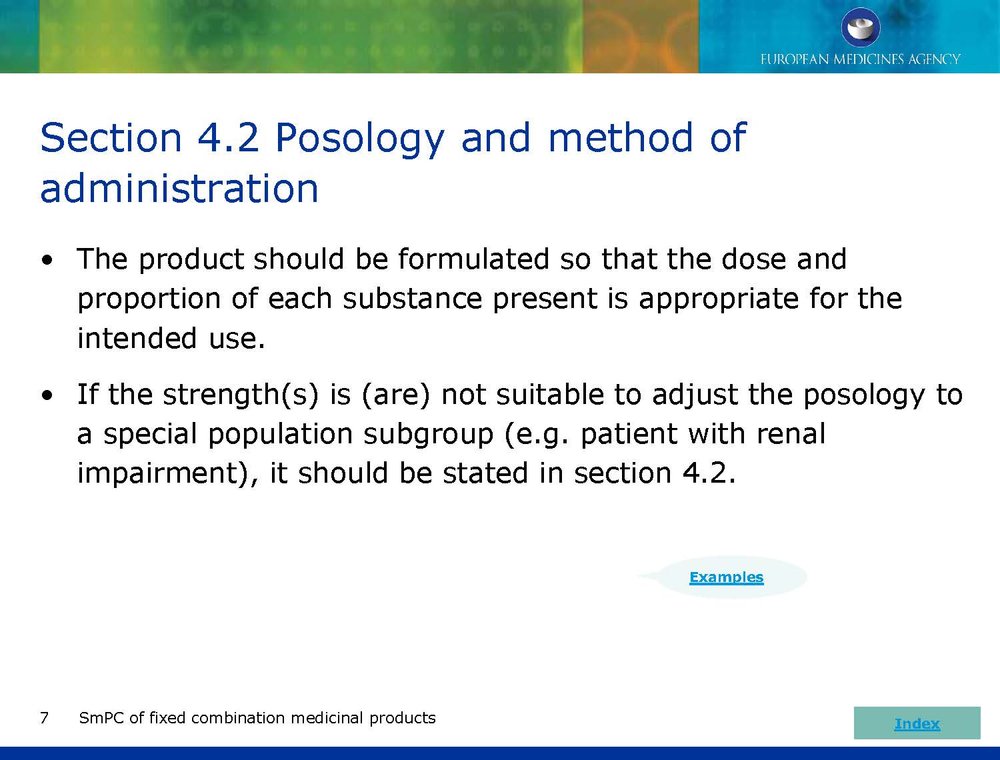
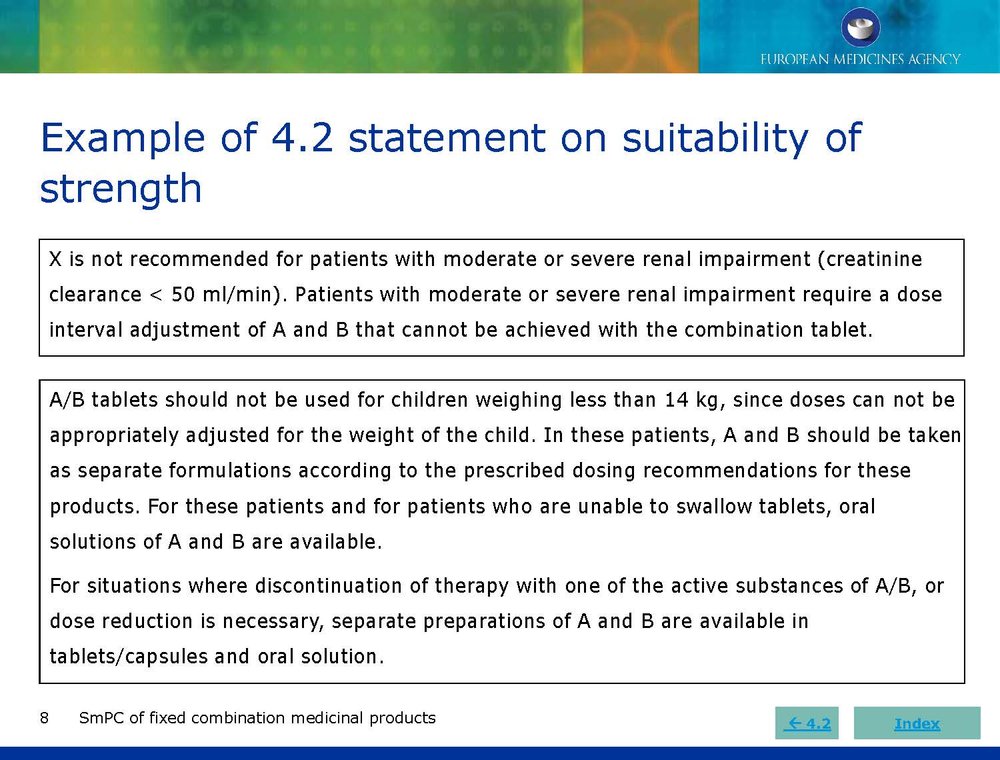

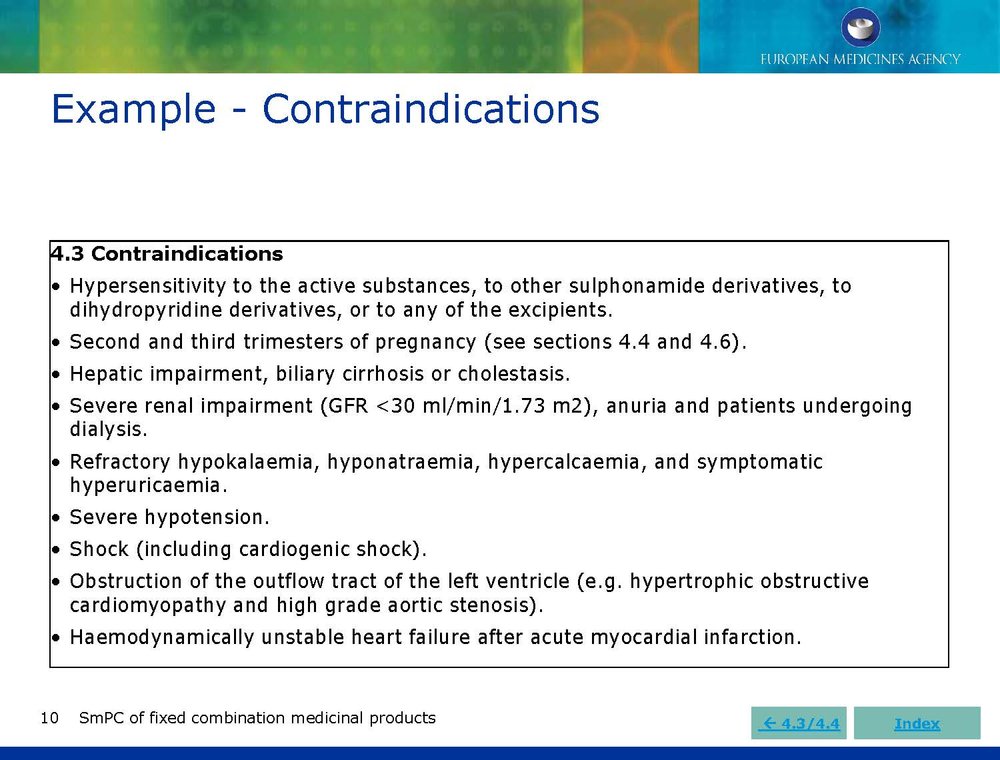


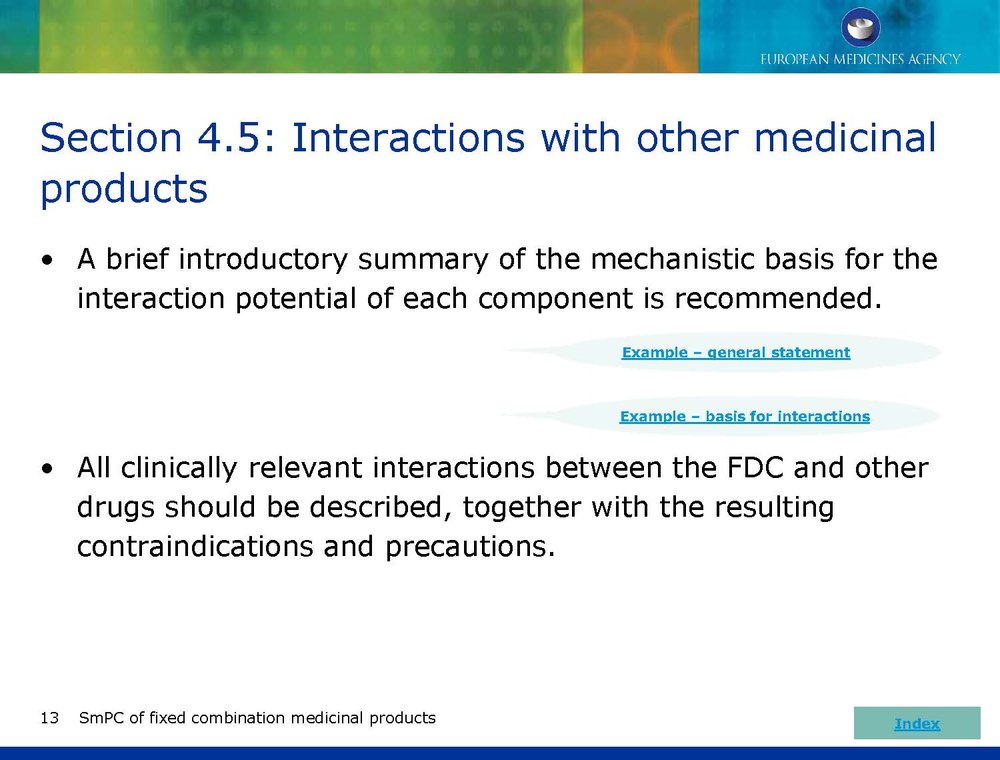
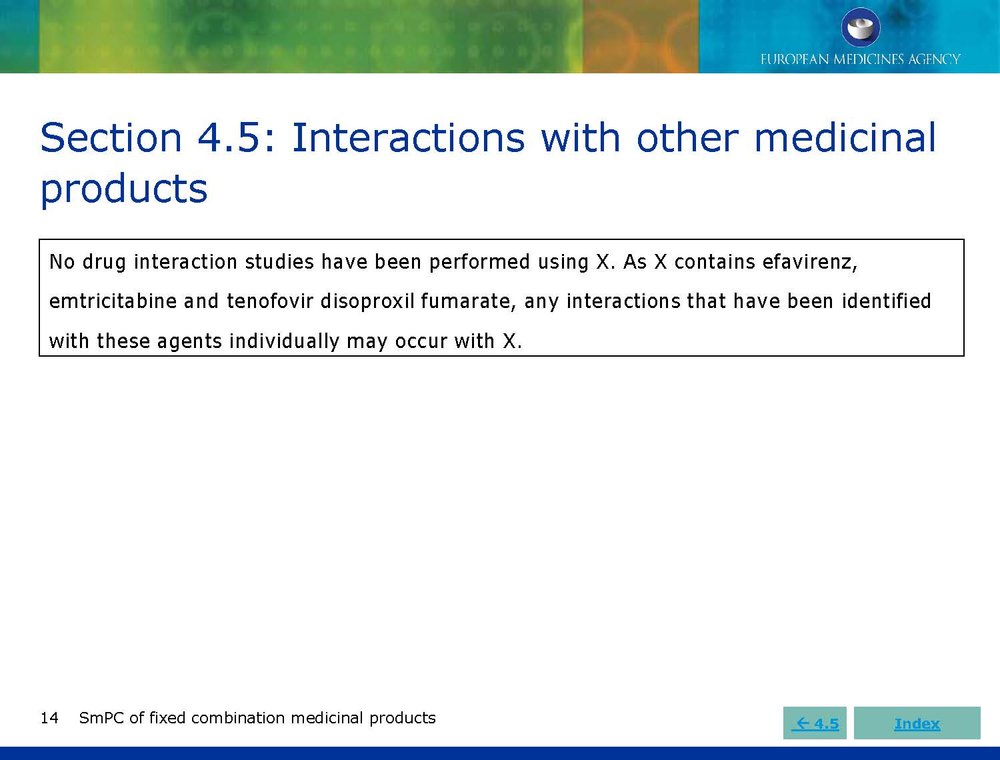
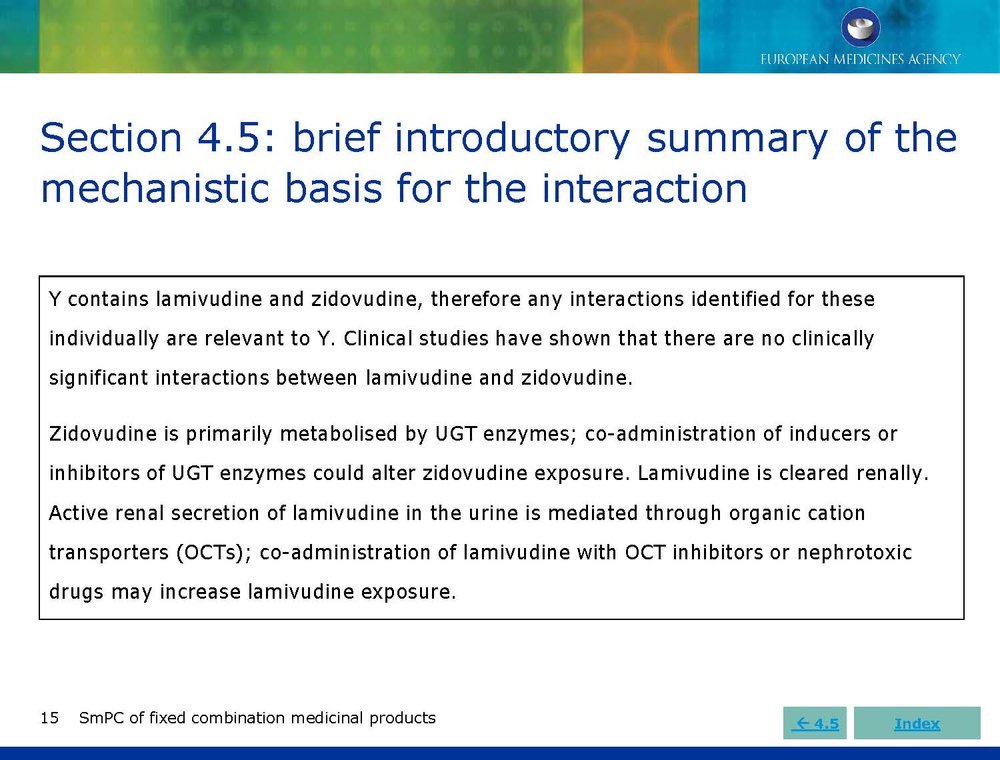


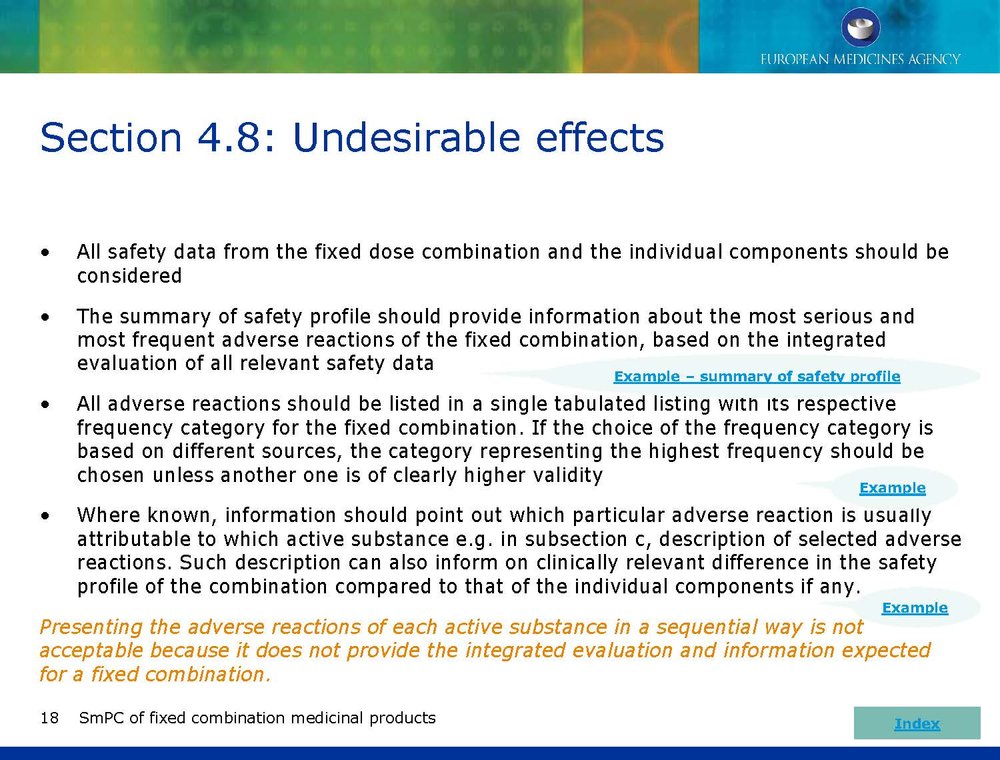
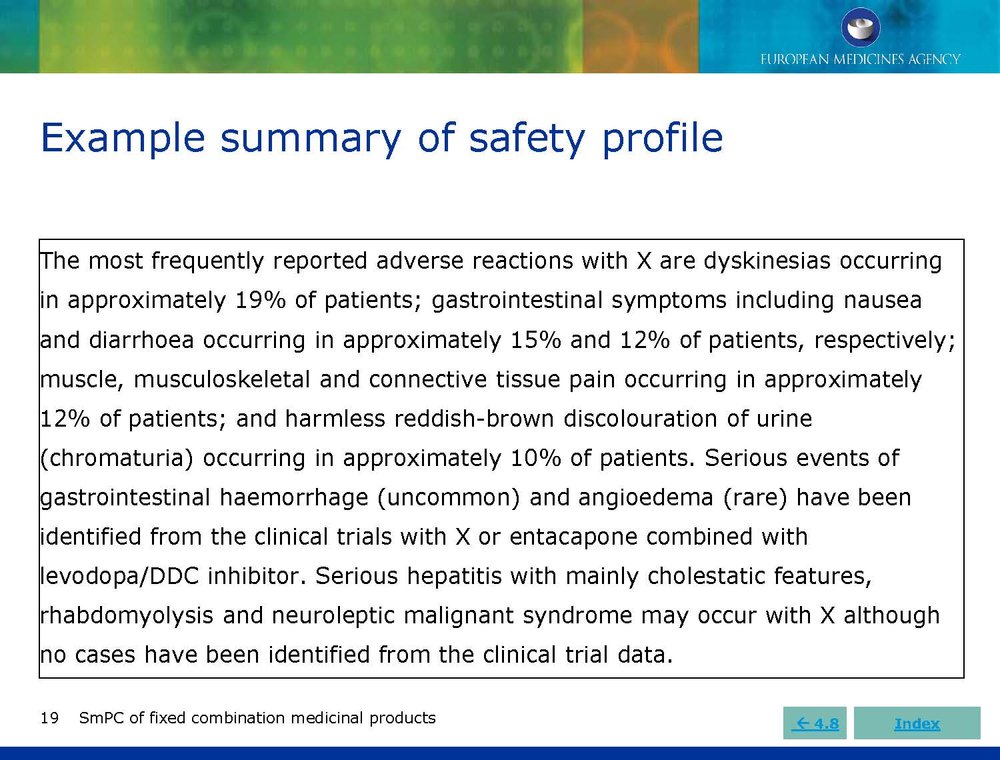
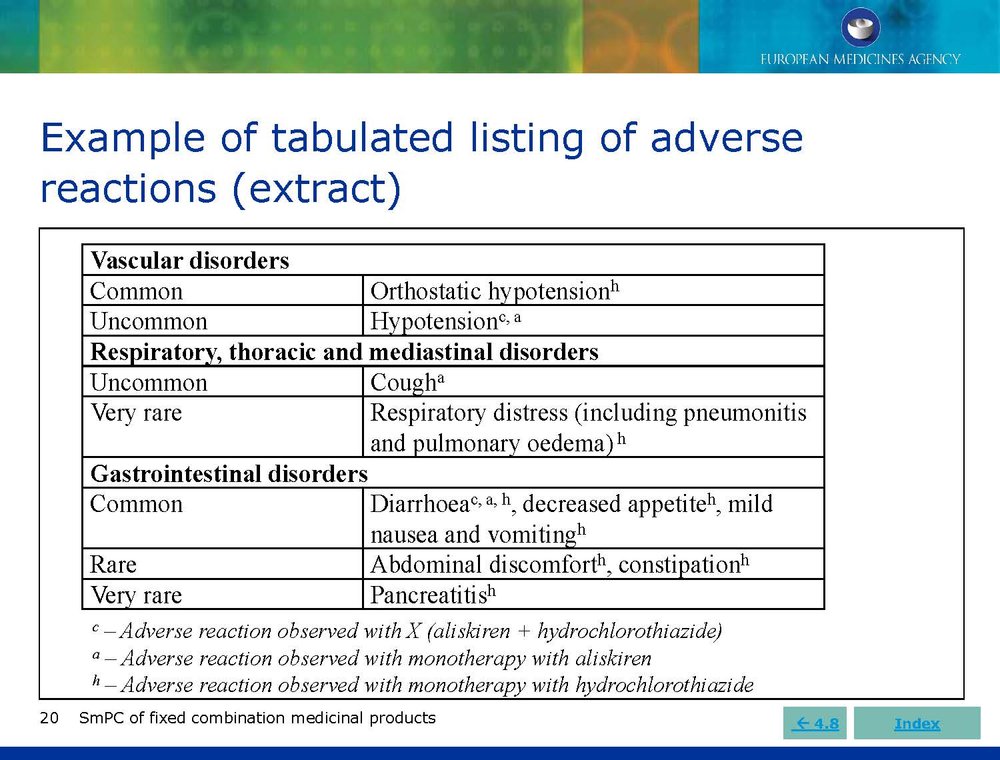
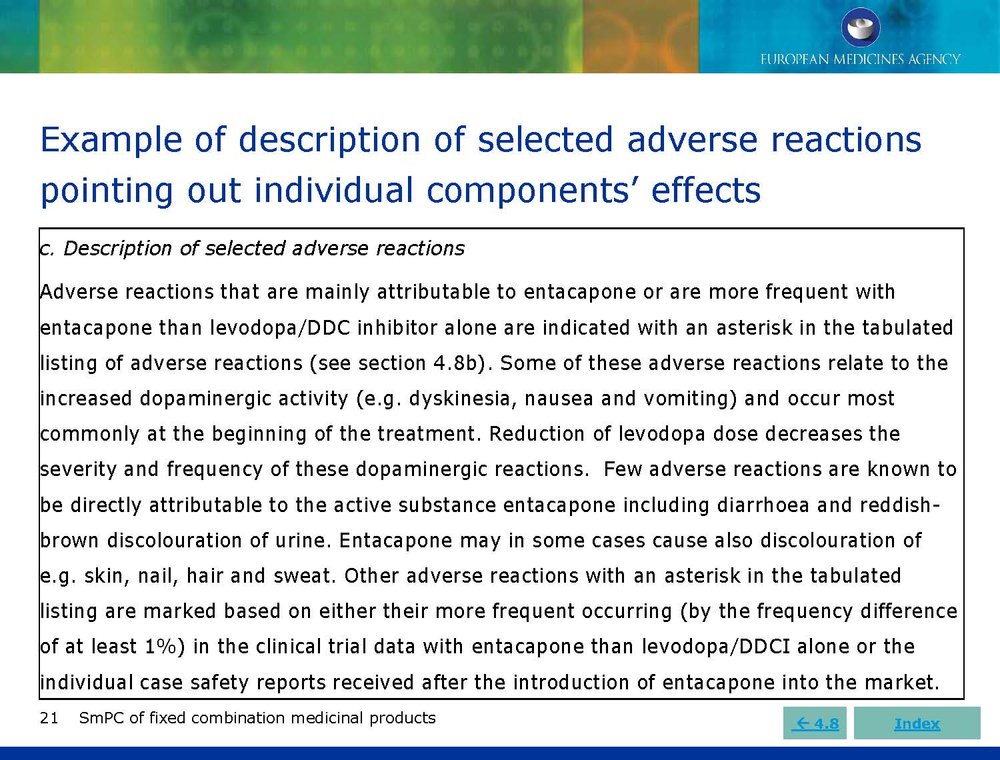
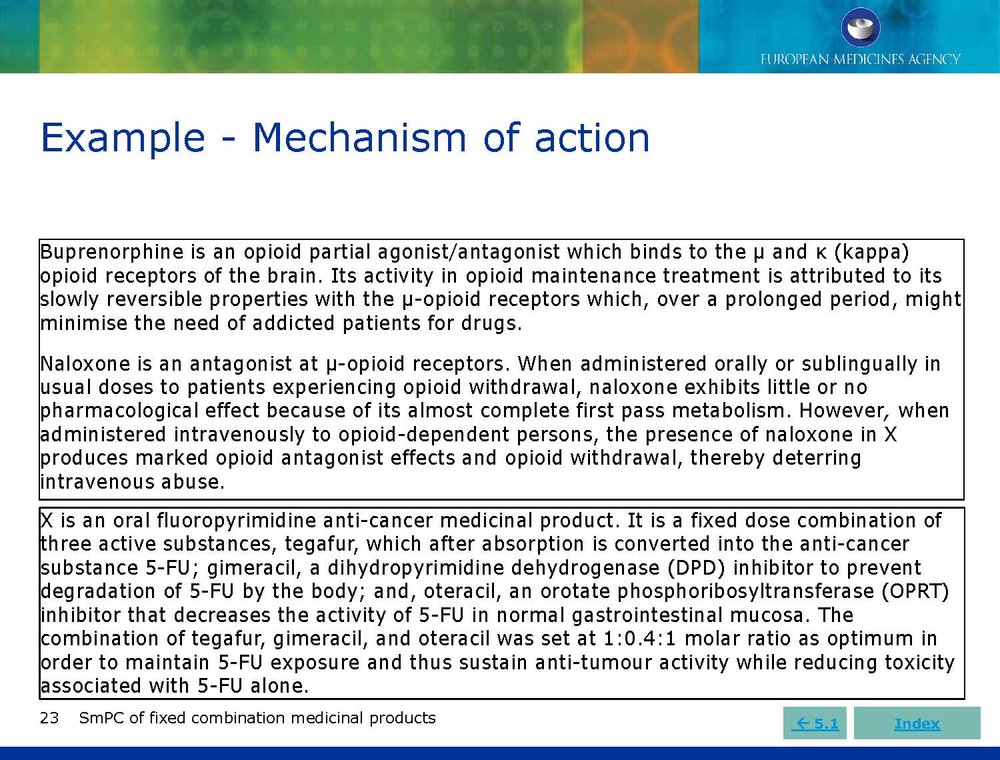
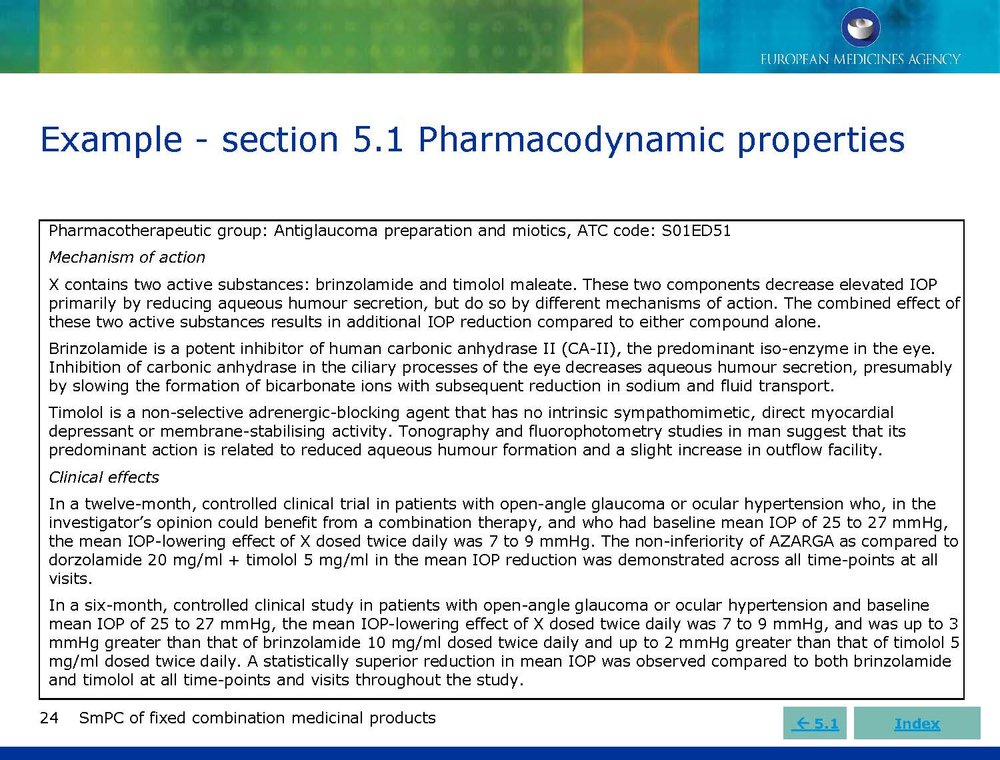
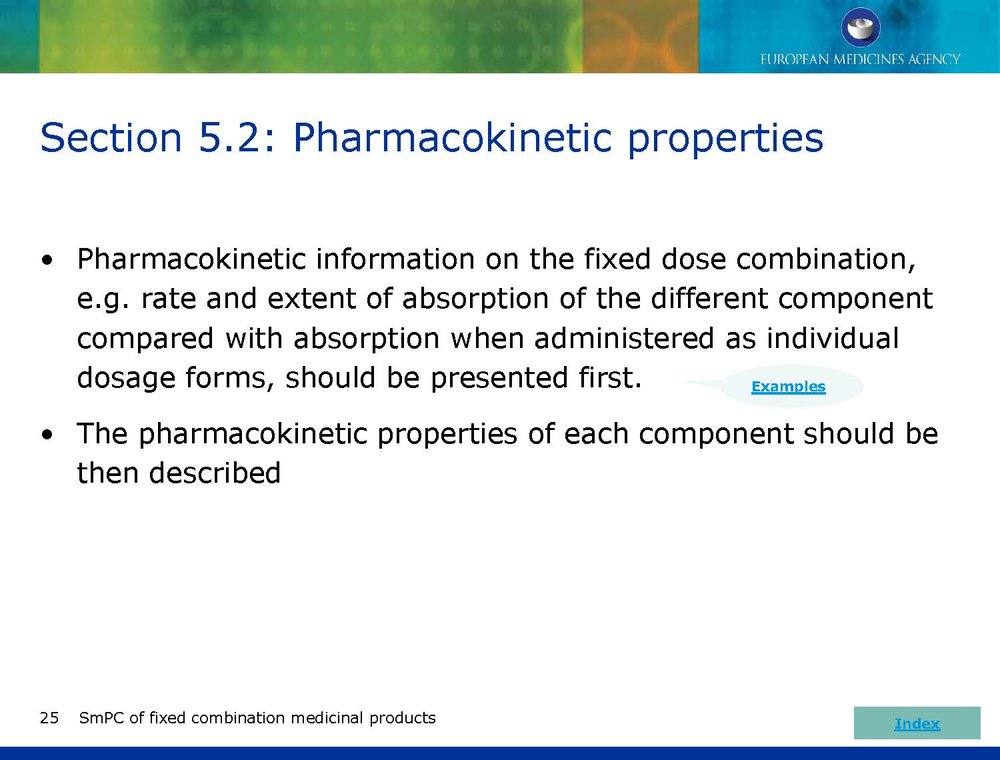
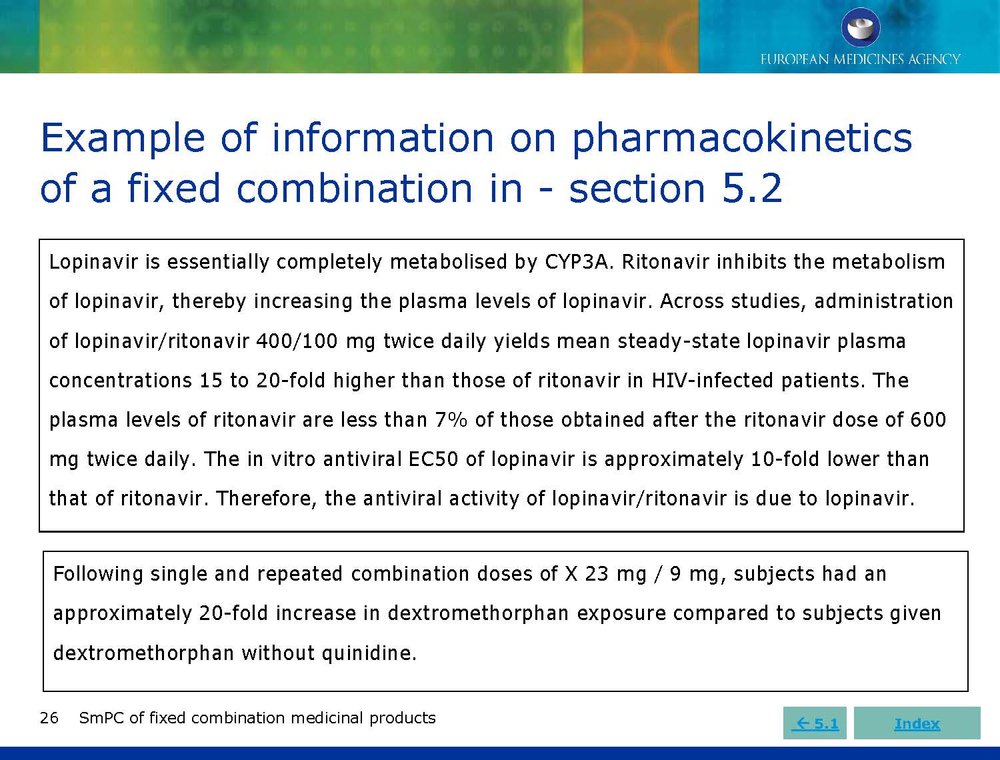

Published EMA Slideshow presentation containing 28 slides about general principals, advice and examples for presenting information in the SmPC of fixed combination medicinal products